Integrated Driver-Based Planning for Banks
The uncertainty of today’s fast-paced world is requiring the planning process to evolve to an …

The implementation of clinical trials is associated with immense costs for the performing company as the trials may be spread over long periods and it is unclear if the tested substance delivers the desired results. To minimize the consequential insecurities and risks it is important to be as accurate as possible in the initial planning and budgeting process. Moreover keeping track of the actual cost development throughout the trial and estimating the further cost progress is of great importance. Our application enables planners to analyze the impact of several cost drivers, such as the number of sites and patients on the total costs to optimize the trial set up. In addition the budgeting process is simplified by allowing planners to refer to actual data of comparable studies that have already been carried out.
A solution by KPMG Germany

The application is split into two sections. Section one concerns the forecasting of trials that have already passed the initial budget process and are currently implemented. Section two considers the budgeting process of new trials.
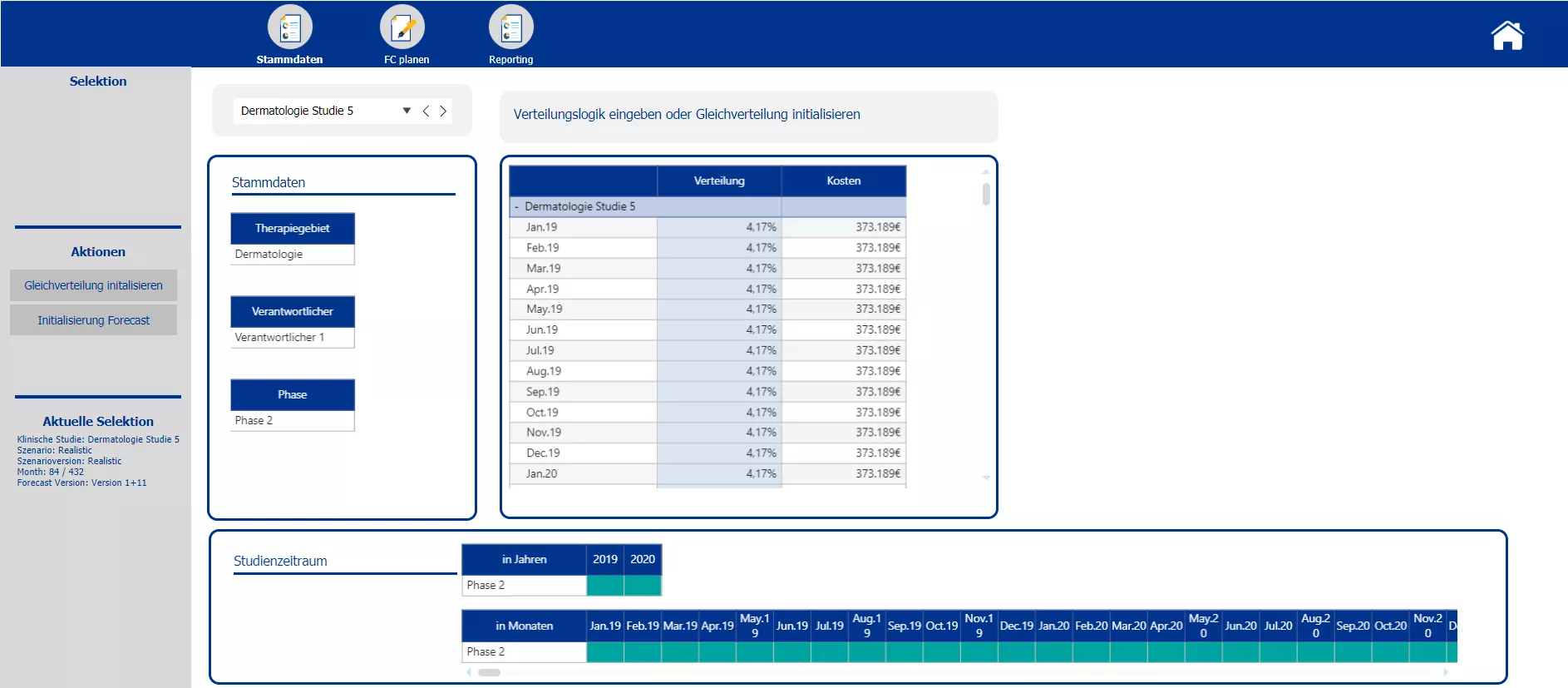
In the first step of the forecasting section planners are enabled to split the initial total budget costs occurring over the whole period of the trial among the individual months. Either a uniform distribution or an individually determined distribution key can be applied. After the initialization of the forecasting, actual data for past months can be uploaded. Overwriting of the forecast values is only possible for upcoming months, data entry is locked for those months that already contain actual data. For every month passed a new forecast version may be implemented to have a continuous forecast. A comparison of the forecasted total costs and the initial total costs, shall help decision makers to steer and make adjustments to the trial set up accordingly. The sketch of a reporting screen provides an idea how the data could be visualized.
Section two starts with the creation of the new clinical trials and the definition of the master data – such as the phase, the responsible person, the medical field and the implementation period. Based on the master data the planner is automatically provided with possible reference studies. All relevant information of the reference trials is presented. To plan the costs a pre-initialization with the data of either one study or the average of all chosen reference studies is possible. A color code indicates pre-initialized cost drivers and blocks. After planning the cost drivers and personal costs, the total costs are calculated. After completing the budgeting, the initial budget can be locked via the workflow. Moreover the workflow allows to reopen the budgeting process of a specific study and provides an overview about the costs and the current process status.
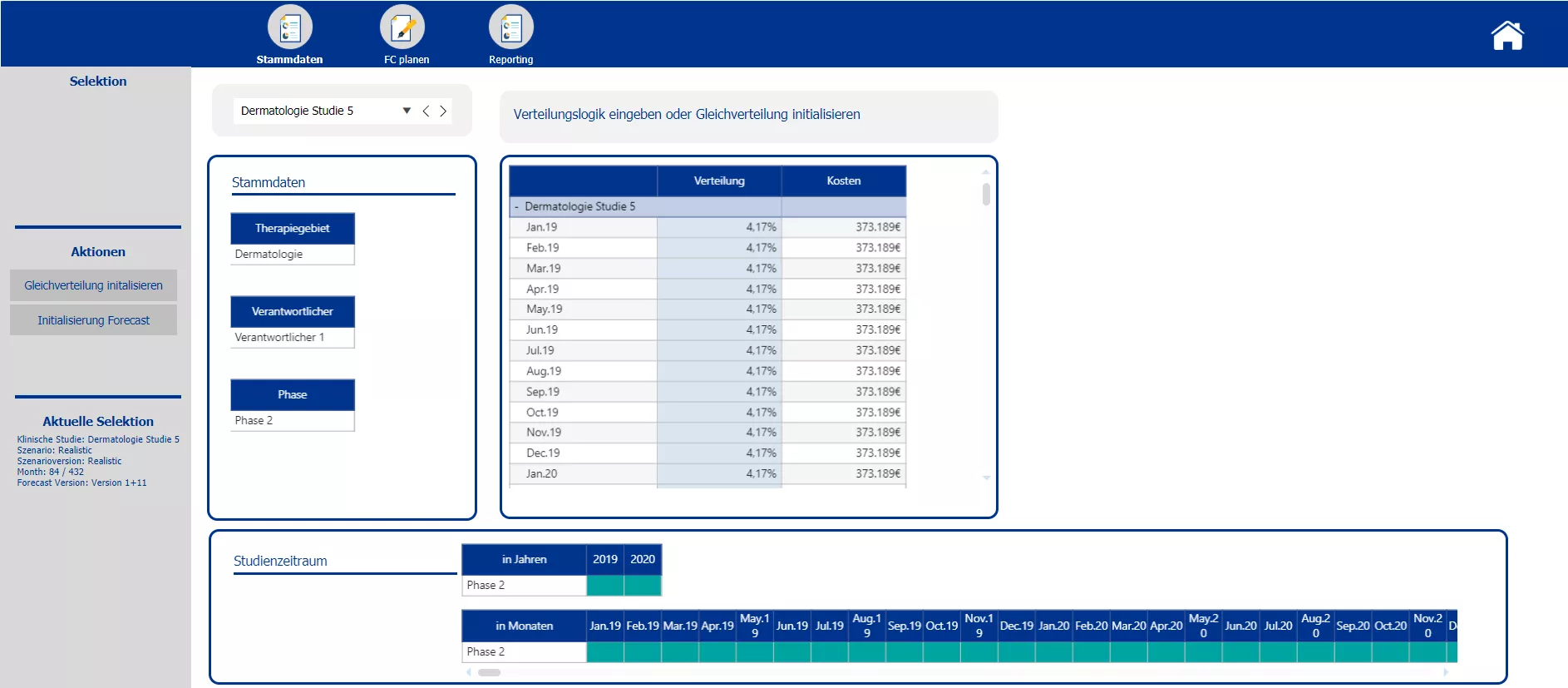
Initialization of a rolling forecast with according logic to split total costs among months
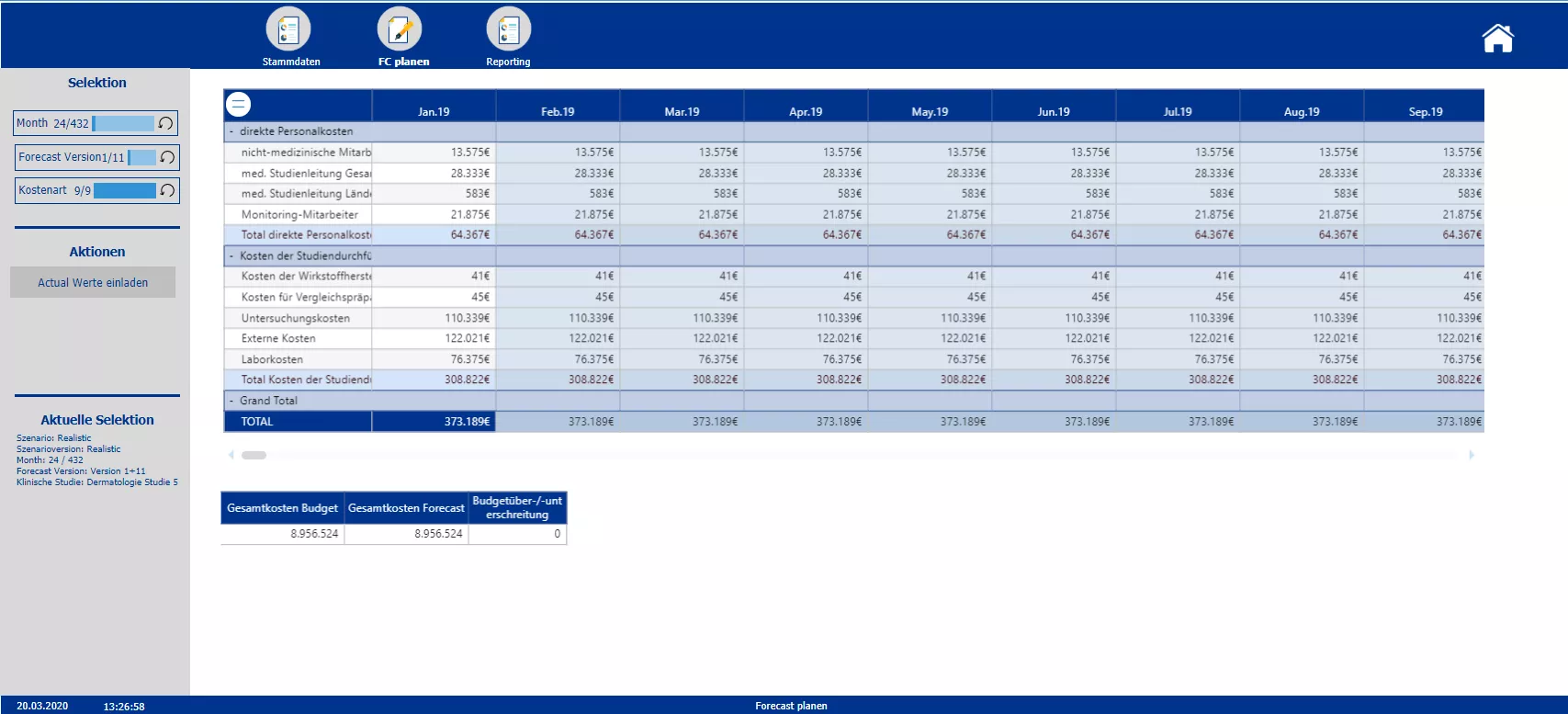
Monthly overview of actual and forecast data. Enabled data entry for future months and overview of initial budget vs. current forecast budget.
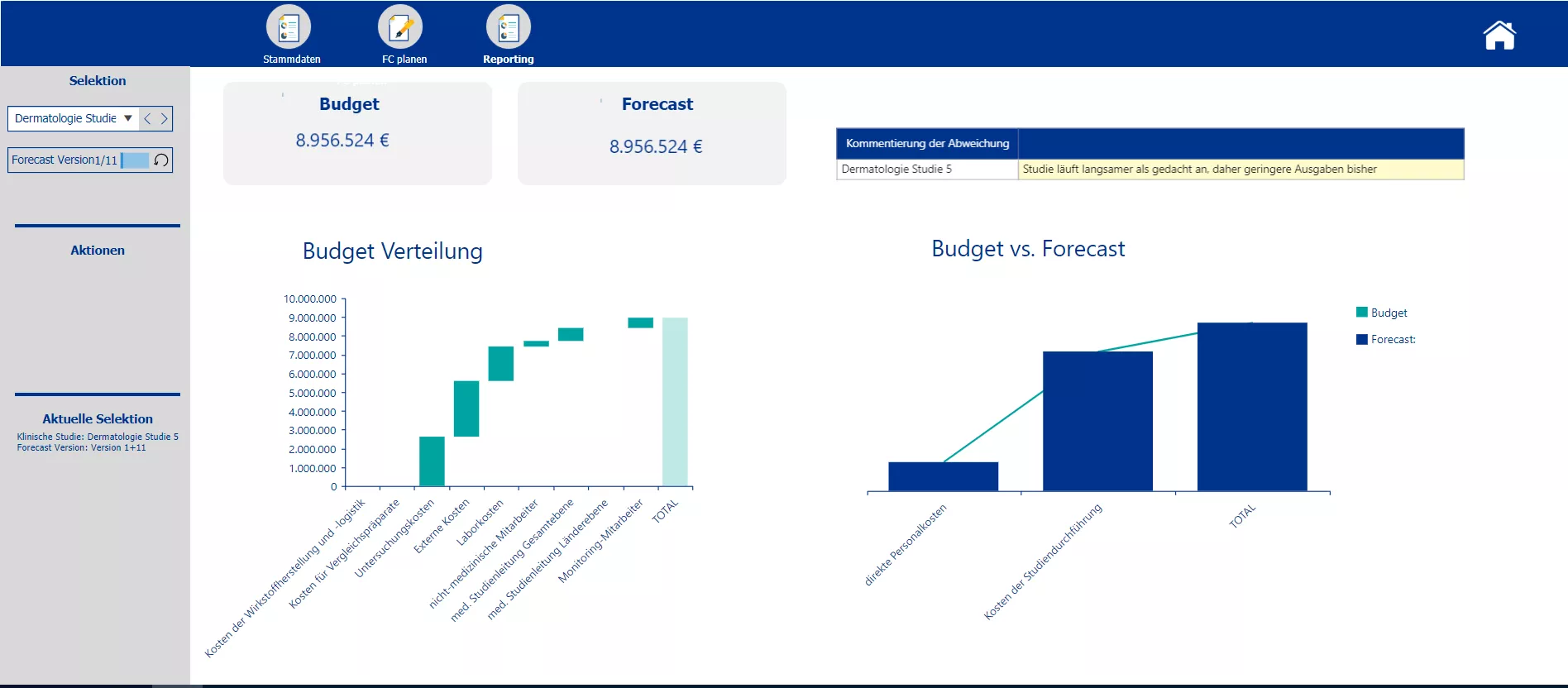
Reporting Screen with division of costs among several cost blocks, comparison of specific cost blocks of the initial and forecast budget. Commenting function for differences of budget and forecast.
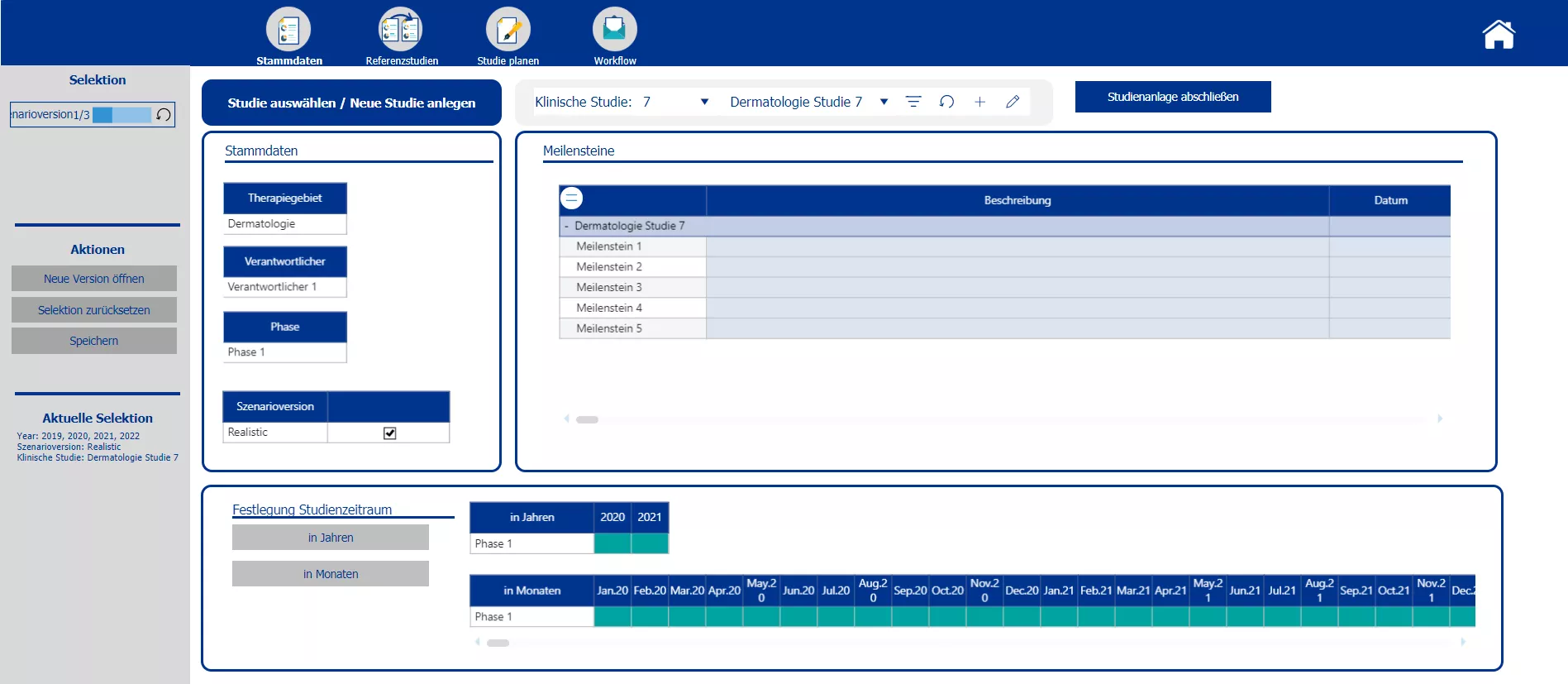
Creation of new studies and the master data, determination of mile stones and selection of the planning scenario.
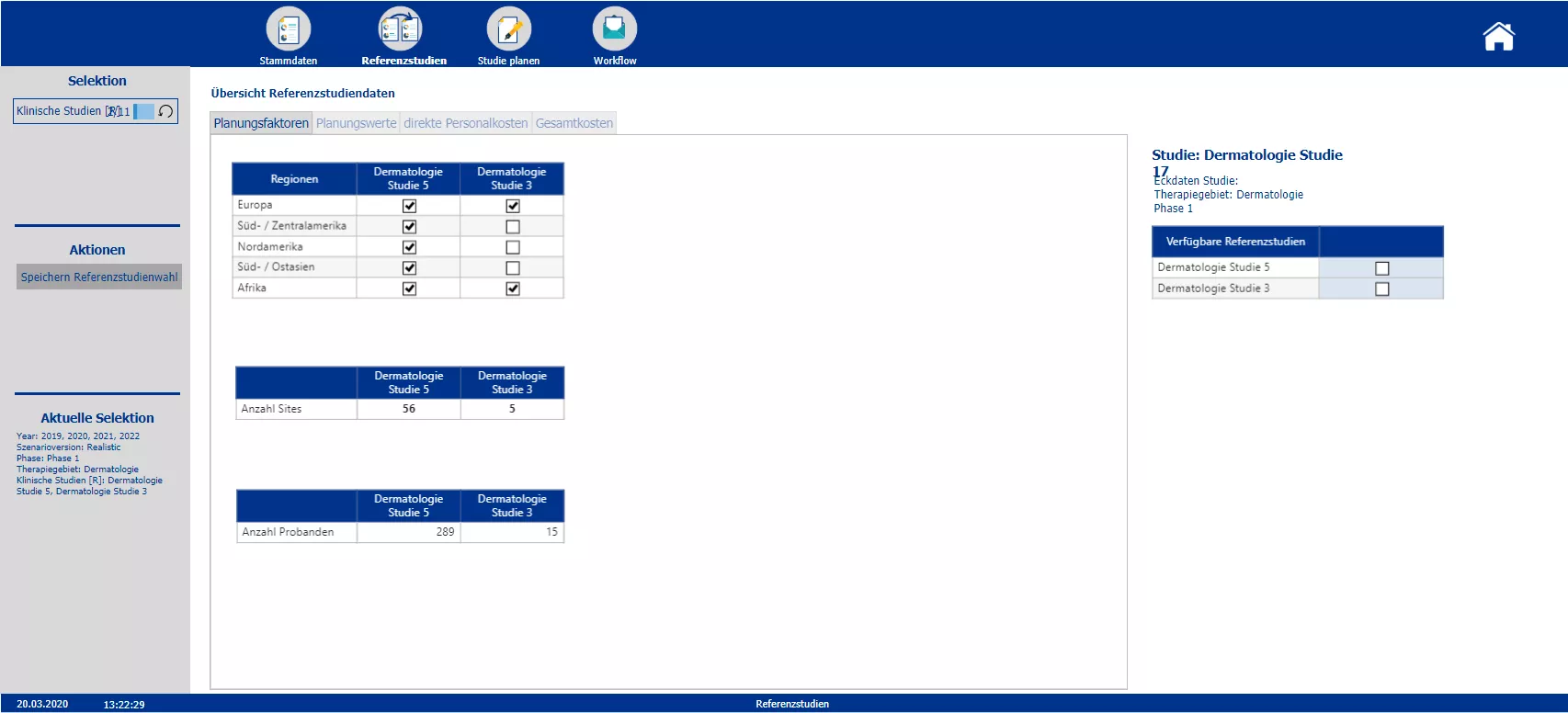
Overview of the costs of possible reference studies and selection of the reference studies to be used in the further process
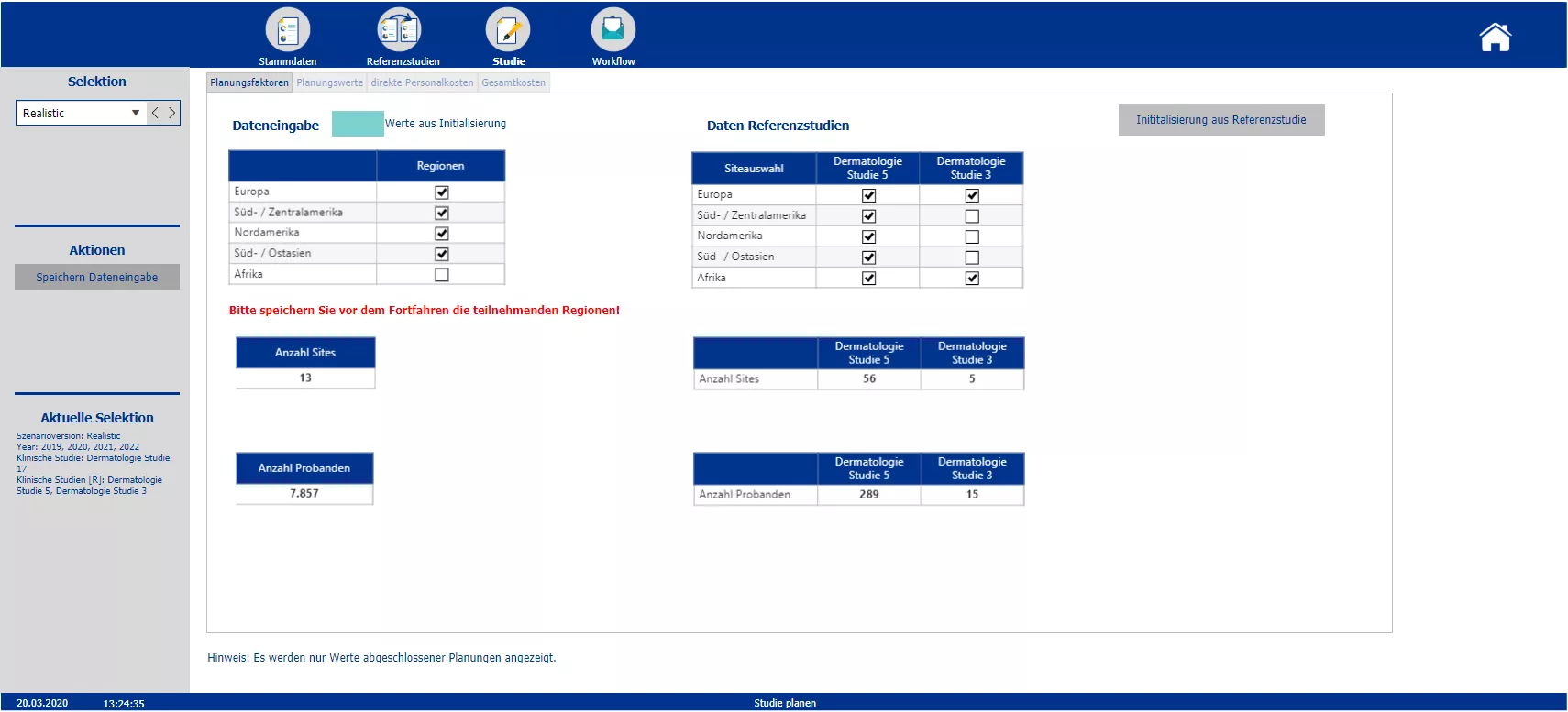
Planning of the trial and possibility to initialize the different cost blocks with data of the chosen reference studies. Color coding of initialized blocks.
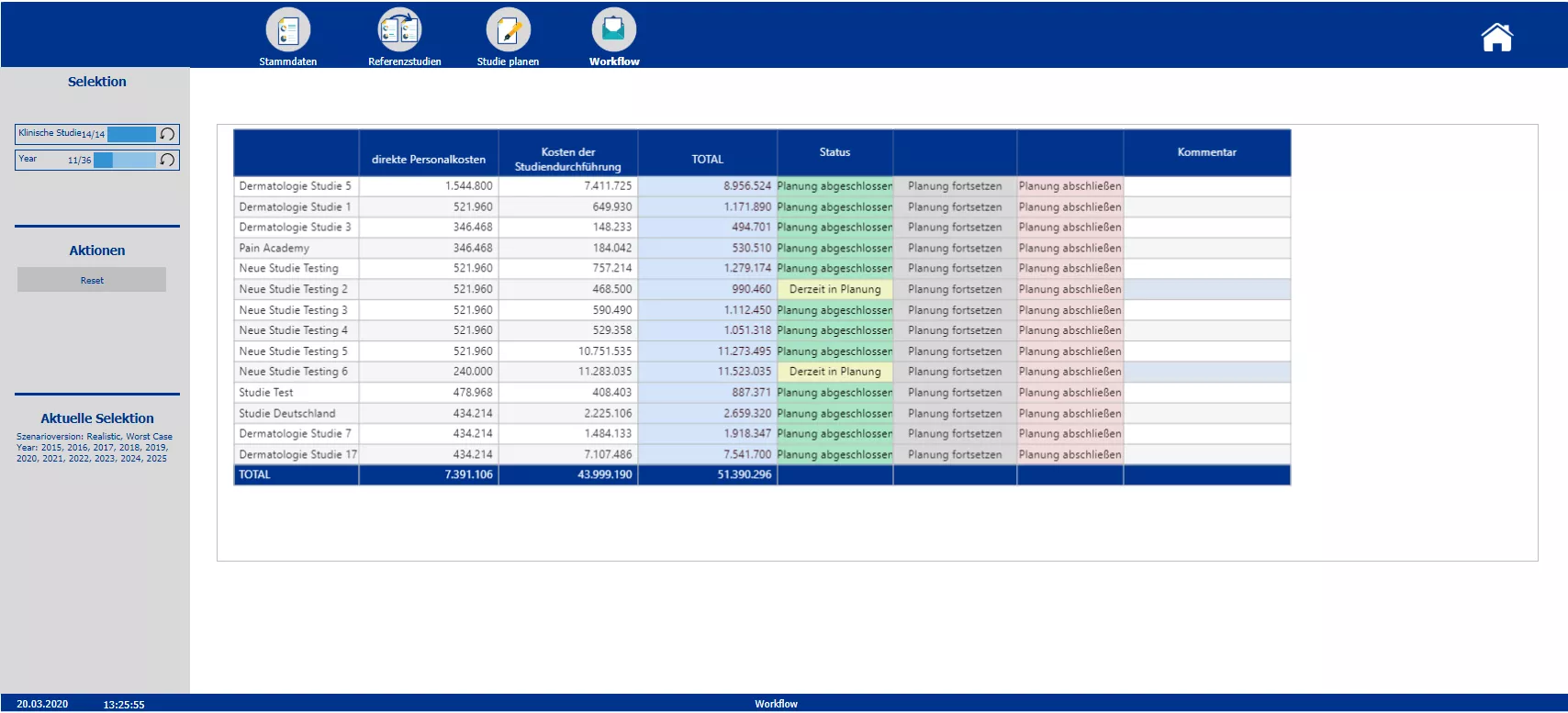
Workflow giving an overview about total costs, status of the budgeting process and possibility to close or continue the budgeting process
The uncertainty of today’s fast-paced world is requiring the planning process to evolve to an …

Both during and after times of crisis, such as COVID 19, companies may find several reasons for …

Successful growth in companies is often linked to a multitude of initiatives that present project …

The topic of Environmental, Social & Governance (ESG) is becoming increasingly important for …
